One of the challenges that the company is facing is finding partners in the health ecosystem. The other one is going through the health regulatory process as each country has its regulatory authority. Sri Lanka’s regulatory authority is currently reviewing the Jendo system. Once the review is completed, it will allow the commercialization of the device. The company will also apply for the United States Food and Drug Administration (FDA) approval, followed by other countries.
Jendo Innovations is also in discussion to establish two business centers outside of Sri Lanka; one in Switzerland, and the other one in Singapore. “Both countries have a favorable business environment, and growth opportunities,” according to Keerthi.
Tried and Tested on Patients to Prevent Heart Disease
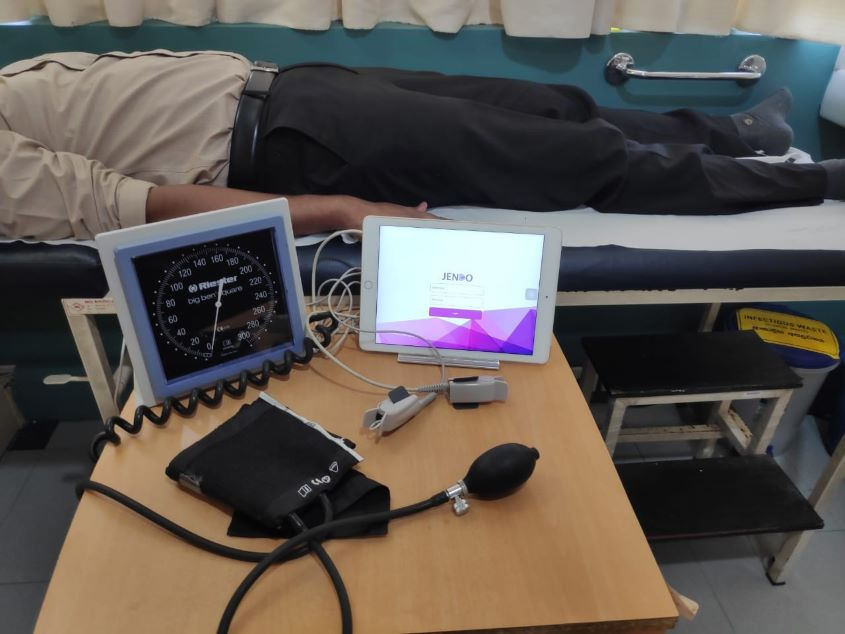
Jendo’s system is not commercialized yet but the device is already being used by hospitals and laboratory chains, and clinically tested on over 800 patients.
Under the current business model, the device will be sold at US$2,250 with an annual subscription fee of US$10,000. The test will be charged US$8. According to Keerthi, “the screening price is affordable in comparison with other established procedures.”
The company swiftly proceeded to apply for a patent, first in Sri Lanka (pending), then in the United States (granted: USPTO Patent 10,912,464 B2), and in Japan (Pending), through the WIPO Patent Cooperation Treaty (PCT).
According to Keerthi, investors want to make sure the products they invest in is ahead of the competition. “A patent is key to proving the value of your invention and winning investors’ confidence,” he said.


